This theory is based on two simple postulates,
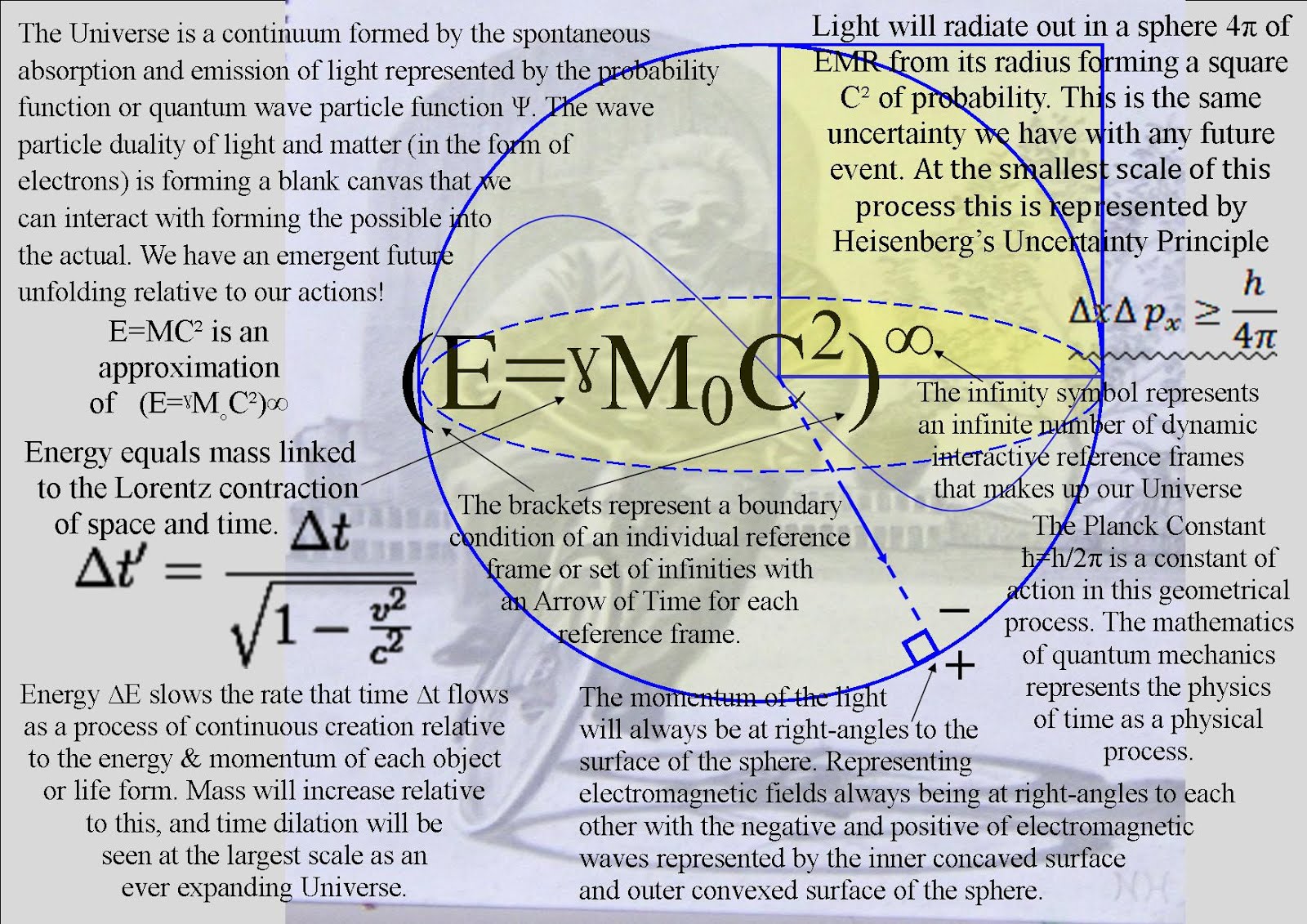
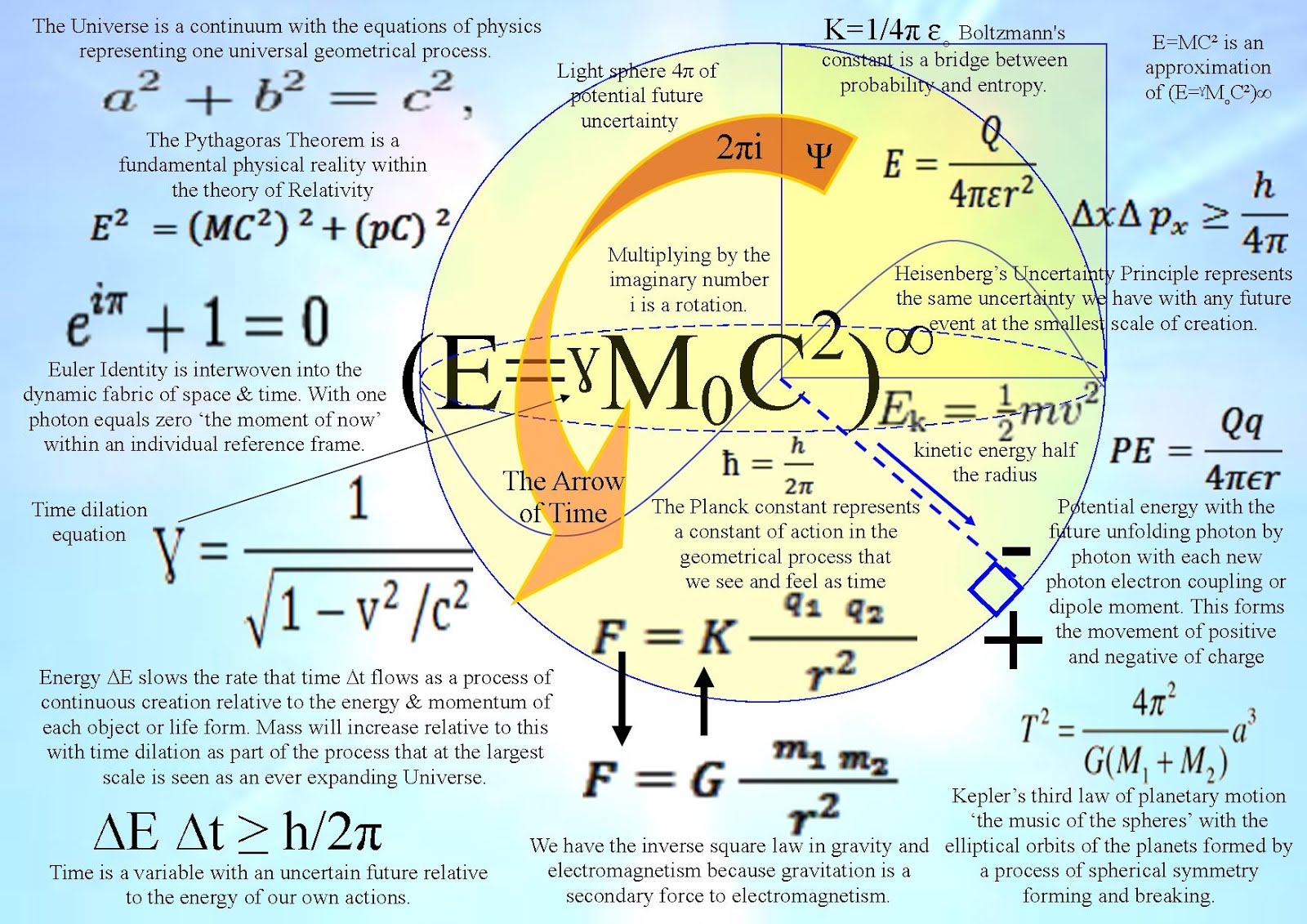
1. The first is that the Quantum Wave Particle Function explained by Schrödinger’s wave equation represents the forward passage of time or arrow of time ∆E ∆t ≥ h/2π itself photon by photon, quanta by quanta or moment by moment.
2. The second is that Heisenberg’s Uncertainty Principle ∆×∆p×≥h/4π that is formed by the Quantum Wave-Particle Function is the same uncertainty that we have with any future event.
~
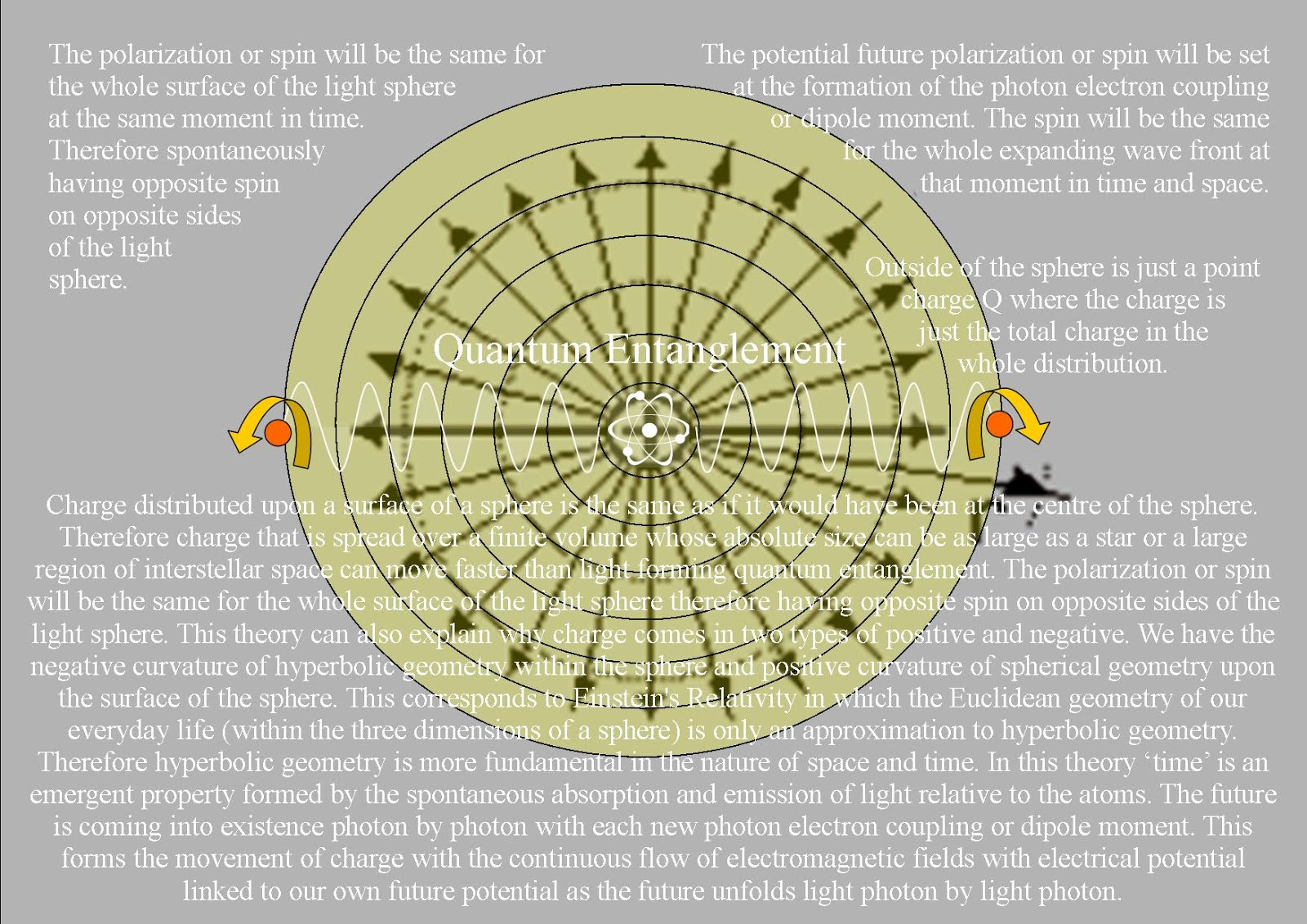
 In Quantum Atom Theory it is random irrational numbers that represent the chaos and probability of everyday life. Space and time are linked as in Einstein’s theories of relativity. This theory explains a process that forms Einstein’s curvature of spacetime. Therefore we have the irrational number π in the equations for the Uncertainty Principle when (time ∆E ∆t ≥ h/2π) and where (position ∆×∆p×≥h/4π) will always be uncertain just like any future event in our everyday life!
In Quantum Atom Theory it is random irrational numbers that represent the chaos and probability of everyday life. Space and time are linked as in Einstein’s theories of relativity. This theory explains a process that forms Einstein’s curvature of spacetime. Therefore we have the irrational number π in the equations for the Uncertainty Principle when (time ∆E ∆t ≥ h/2π) and where (position ∆×∆p×≥h/4π) will always be uncertain just like any future event in our everyday life!
~
Link to more videos on Quantum Atom Theory
~